Pioneering the future of MedTech compliance through innovation
MedTech compliance is broken
Today, manufacturers in MedTech face huge challenges: fragmented systems, manual processes, static documents, and reactive compliance cultures.
These obstacles slow down innovation, drive up costs, and ultimately delay patient access to life-saving technologies.
That need to change.
And that is why Pixcelium is building the first end-to-end MedTech compliance ecosystem.
The first E2E ecosystem
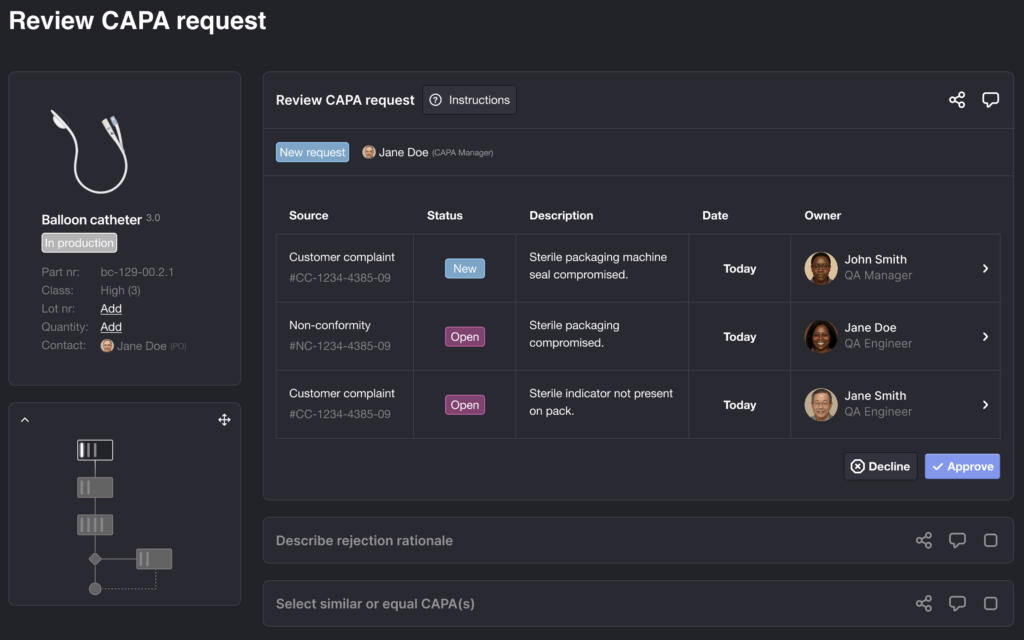
We are building the first end-to-end compliance ecosystem for MedTech, one that replaces siloed, document-driven workflows with structured, process-first, data-driven operations.
From CAPA and Risk Management to Technical Files and Post-Market Surveillance, Pixcelium creates a living compliance backbone that grows with your organization.
This isn’t about digitizing paperwork. It’s about creating a connected foundation where compliance supports decision-making, accelerates audits, and empowers teams to innovate with confidence.

Our inspiration comes from nature itself.
Just as mycelium forms a living network beneath the forest floor, connecting and nourishing entire ecosystems, Pixcelium connects compliance data, processes, and people into one intelligent, adaptive environment. Information flows seamlessly, enabling traceability, transparency, and proactive quality by design.
Human centric compliance
Compliance should feel human. Not a maze of files or a wall of acronyms, but a clear, supportive flow that helps people do their best work.
We don’t merely ship software; we solve. We enter complex, document-bound environments and leave behind connected, data-driven processes that are easier to run, easier to trust, and ready to scale across teams, sites, and markets.
Our promise is rigorous by design and graceful in experience: turn static documents into living data, then orchestrate the process around it.
Our team
Pixcelium brings together a diverse group of MedTech, regulatory, software, and design professionals. We combine deep technical expertise with a human-centric approach, ensuring that even the most advanced capabilities remain intuitive and accessible.
Our founder, Loïc Brandsma, brings two decades of experience at the intersection of precision engineering, quality management, and regulated manufacturing.
Around him, a team of engineers, clinical and regulatory experts, designers, and strategists share one mission: to transform compliance complexity into clarity.
Where we are today…
Pixcelium is currently working with select pilot customers, shaping and validating our prototype with real-world use cases. Together, we are proving that compliance can be seamless, intelligent, and connected.
With strategic partnerships, growing traction across Europe, and an ambitious roadmap, Pixcelium is laying the foundation for a new standard in compliance, one that keeps pace with regulatory demands while unlocking the full potential of MedTech innovation.

Stay connected
Join Pixcelium
The industry game changer
Curious how Pixcelium is redefining compliance in MedTech?
Our newsletter offers you a front-row seat.
Delivered straight to your inbox, clear, concise, and only when it matters.
You’ll receive:
Insights into how we solve systemic compliance challenges
Early access to our vision, tools, and development roadmap
A behind-the-scenes look at the team and philosophy shaping Pixcelium
Invitations to upcoming product sessions, events, and pilot opportunities